简介
Introduction
弧菌属( Vibrio spp.) 是一类广泛分布于海洋环境中的革兰氏阴性细菌(Baker-Austin C et al., 2017)。海洋弧菌广泛分布于内湾、沿岸、外洋水域、沉积物和海洋生物体中,是一类广为人知的机会致病菌(opportunistic pathogen)。因弧菌感染引发的弧菌病(vibriosis)使我国海洋渔业蒙受巨大经济损失,并对海洋生态环境的可持续发展构成了负面冲击(谢珍玉等, 2005; Grimes, 2020)。弧菌还能通过被其污染的各种海产品经由食物链感染人类,对人类健康造成严重威胁。海洋弧菌被认为是水产养殖业发展的重要限制性因素(夏凡等, 2011)。最为常见的病原弧菌种类是哈维氏弧菌( V. harveyi ) 、副溶血弧菌( V.parahemolyticus)和溶藻弧菌(V. alginolyticus)、创伤弧菌(V. vulnificus)和霍乱弧菌(V. cholerae)等。溶藻弧菌与哈维氏弧菌、副溶血弧菌并称海洋三大优势弧菌,其中溶藻弧菌居首。
Vibrio spp. are a group of Gram-negative bacteria widely distributed in the marine environment (Baker-Austin C et al., 2017). Marine Vibrio is widely distributed in inlets, coastal and pelagic waters, sediments and marine organisms, and is a widely known opportunistic pathogen. Vibriosis caused by Vibrio infections has caused huge economic losses to marine fisheries in China and posed a negative impact on the sustainable development of marine ecosystems (Zhenyu Xie et al., 2005; Grimes, 2020). Vibrio can also infect humans through the food chain by contaminating various seafood products, posing a serious threat to human health. Vibrio maritimus is considered to be an important limiting factor for the development of aquaculture (fan Xia et al., 2011). The most common pathogenic Vibrio species are V. harveyi, V. parahemolyticus, V. alginolyticus, V. vulnificus, and V. cholerae. V. alginolyticus, V. harveyi and V. parahaemolyticus are the three main species, of which V. alginolyticus is the first.
病原性海洋弧菌的主要种类
The main species of pathogenic marine vibrios
(1)哈维氏弧菌
(1) V. harveyi
哈维氏弧菌(V. harveyi)是一种普遍存在于海洋和河口生态系统中的致病性弧菌,是影响热带和亚热带海水养殖动物的最常见病原,对包括我国在内的全球的海水养殖业造成了严重危害(Zhang XH et al., 2001)。哈维氏弧菌菌体呈杆状或短杆状,具有单生鞭毛,有的会出现弯曲(图1 A 和D),其次研究表明哈维氏弧菌具有发光现象(图1 B),并且其在TCBS 培养基上菌落为黄色(图1 C)。感染弧菌病原的患病死亡鱼类出现张口、鳞片脱落、鳍条溃烂和体表溃疡等主要症状,尤以尾鳍溃烂最为严重(梅冰等, 2010)。据不完全统计,近三年来,仅对虾苗种一项,就给海南造成了几十亿元的直接经济损失。因此,制定有效措施预防养殖鱼类和渔业工人感染哈维氏弧菌具有重要意义。目前已经确定的弧菌毒力基因主要包括毒力因子基因(溶血素、蛋白酶、脂多糖等)、营养夺取基因、菌体运动与定居基因、环境感应基因和密度感应基因等(谢珍玉等, 2005)。
V. harveyi is a pathogenic vibrio commonly found in marine and estuarine ecosystems, and is the most common pathogen affecting tropical and subtropical mariculture animals, causing serious harm to mariculture industries worldwide, including China (Zhang XH et al., 2001). V. harveyi is rod-shaped with monotrichous flagella, some of which are curved (Fig. 1 A and D). It has been shown to be luminescent (Fig. 1 B), and its colonies are yellow on TCBS medium (Fig. 1 C). The main symptoms of dead fish infected are mouth opening, scale loss, fin ulceration and body ulceration, especially caudal fin ulceration (Bing Mei et al., 2010). According to an incomplete statistic, in the past three years, only in term of the shrimp fry, the V. harveyi has caused direct economic losses of billions of yuan in Hainan. Therefore, it is important to develop effective measures to prevent V. harveyi infection in farmed fish and fishery workers. The identified virulence genes of V. harveyi include virulence factor genes (hemolysin, protease, lipopolysaccharide, etc.), nutrient seizure genes, thallus movement and settlement genes, environmental sensing genes and density sensing genes (Zhenyu Xie et al., 2005).
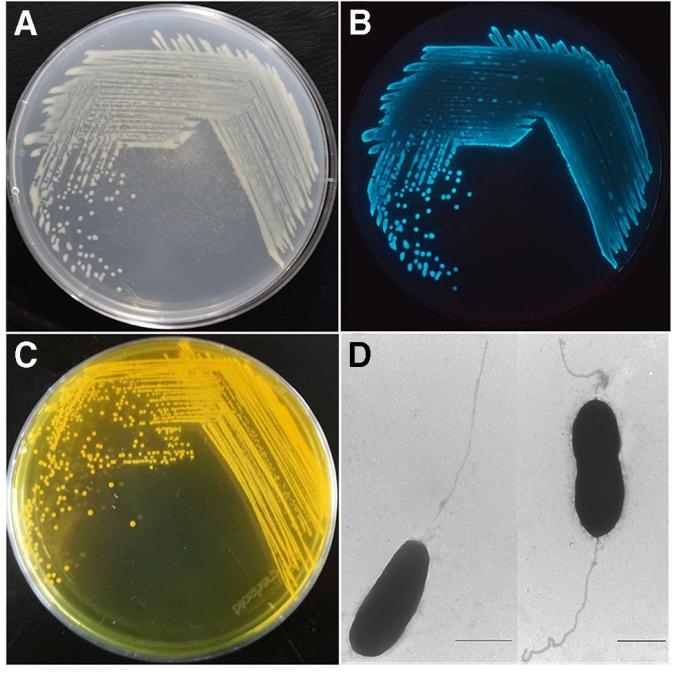
图1. 哈维氏弧菌菌株的形态(Zhang Xiao-Hua et al., 2020)。A 图示V. Harveyi VIB391 菌株在海洋2216E 培养基上的生长形态;B 图示V. Harveyi VIB 391 菌株的发光现象;C 图示V. Harveyi VIB 645 菌株在TCBS 培养基上的生长形态;D 图示V. Harveyi VIB 645 菌株在透射电镜下的形态。
Fig. 1. Morphology of V. harveyi strains (Zhang Xiao-Hua et al., 2020). A. Growth pattern of V. harveyi VIB391 on marine 2216E medium; B. Luminescence of V. harveyi VIB 391; C. Growth pattern of V. harveyi VIB 645 on TCBS; D. The morphology of V. Harveyi VIB 645 under transmission electron microscope.
(2)副溶血弧菌
(2) V. parahemolyticus
副溶血弧菌(V. parahaemolyticus)广泛分布于海洋环境中,可以导致水生动物发病,如对虾急性肝胰腺坏死病,给水产养殖带来严重的经济损失(俞盈等,2012),感染的对虾表现出具有浑浊的肝胰腺,并且发现其外骨骼上有棕色至黑色斑点,其排出粪便为白色(图2)。副溶血弧菌感染的典型临床特征包括腹部绞痛、腹泻、恶心、头痛、发烧和发冷(Baker-Austin C et al., 2010);严重情况下,经伤口感染该菌会产生败血症,进而导致死亡。研究发现,食腐质性食物的鱼类体内一般含较多的副溶血弧菌,而草食性鱼类的体内则较少,而且副溶血弧菌多半在鱼的鳃和肠内,皮肤上很少发现。目前已经确定了副溶血弧菌的多种毒力相关因子,主要有热稳定直接溶血素(thermostable direct hemolysin, TDH)、耐热直接溶血相关毒素(TDH-related hemolysin, TRH)、Ⅲ型分泌系统(the type secretion Ⅲ systems, T3SS)和Ⅵ型分泌系统等。在感染过程中,副溶血弧菌利用粘附因子与宿主细胞上的纤维连接蛋白和磷脂酸结合,并利用T3SS1 和T3SS2将不同的效应物和毒素转运到细胞质中,引起细胞毒性和严重疾病(Baker-Austin C et al., 2017)。
V. parahaemolyticus is widely distributed in the marine environment and can cause disease in aquatic animals, such as acute hepatopancreatic necrosis in shrimp, which brings serious economic losses to aquaculture (Ying Yu et al., 2012). The infected shrimp has turbid hepatopancreatic glands and are found to have brown to black spots on their exoskeletons, and their feces are white (Fig. 2). Typical clinical features of V. parahaemolyticus infection include abdominal cramp, diarrhea, nausea, headache, fever, and chill (Baker-Austin C et al., 2010); in severe cases, wound infection with the bacterium can produce sepsis, which can lead to death. V. parahaemolyticus has been found to be more common in fish that eat saprophytic foods, but less so in herbivorous fish, and it is mostly found in the gills and intestines of fish but rarely on the skin. Various virulence-related factors of V. parahaemolyticus have been identified, mainly thermostable direct hemolysin (TDH), TDH-related hemolysin (TRH), the type secretion Ⅲ systems (T3SS) and type secretion VI systems (T6SS). During infection, V. parahaemolyticus uses adhesion factors to bind to fibronectin and phosphatidic acid on host cells and uses T3SS1 and T3SS2 to translocate different effectors and toxins into the cytoplasm, causing cytotoxicity and severe disease (Baker-Austin C et al., 2017).

图2. 来自卡纳塔克邦沿海养殖池塘的感染凡纳滨对虾(Prithvisagar Kattapuni Suresh et al., 2021)。A 图示浑浊的肝胰腺;B 图示白色粪便;C 图和D 图示外骨骼上的棕色至黑色斑点。
Fig. 2. Infected Litopenaeus vannamei from a coastal culture pond in Karnataka (Prithvisagar Kattapuni Suresh et al., 2021). A Turbid hepatopancreas; B White feces; C, D brown to black spots on exoskeletons.
(3)溶藻弧菌
(3) V. alginolyticus
溶藻弧菌(V. alginolyticus)是一种革兰氏阴性条件致病菌,在鱼类、虾类和贝类等多种海洋生物中引起不同程度的疾病。溶藻弧菌引起的弧菌病流行范围广,给养殖业造成了严重的经济损失。除此之外,溶藻弧菌可引起人类胃肠炎、中耳炎、外耳道炎和败血症。目前已经确定了溶藻弧菌的多种毒力因子,其会产生羟肟酸型铁离子团和三种溶血素:热稳定直接溶血素(TDH)、热稳定相关溶血素(TRH)和热不稳定直接溶血素(TLH)(Natividad-Bonifacio et al., 2013; Sha Jian et al., 2013)。研究表明,vppC 基因是溶藻弧菌致病的关键毒力基因。除此之外,粘附、细胞外产物和其他毒力因子也会影响溶藻弧菌的致病性。其中粘附是致病菌的关键毒力因子,也是宿主细菌感染的先决条件。
V. alginolyticus is a Gram-negative pathogenic bacterium that causes varying degrees of diseases in a variety of marine organisms, including fish, shrimp, and shellfish. V. alginolyticus causes widespread vibriosis, which causes serious economic losses to the aquaculture industry. In addition, V. algolyticus can cause gastroenteritis, otitis media, otitis externa and septicemia in humans. Various virulence factors have been identified for V. alginolyticus, which produces hydroxamic acid-type iron ionophores and three hemolysins: TDH, TRH and thermolabile hemomysin (TLH) (Natividad-Bonifacio et al., 2013; Sha Jian et al., 2013). It was shown that the vppC gene is a key virulence gene for V. alginolyticus. In addition, adhesion, extracellular products and other virulence factors also affect the pathogenicity of V. algolyticus. Among them, adhesion is the key virulence factor of pathogenic bacteria, and is also a prerequisite for host bacterial infection.
(4)创伤弧菌
(4) V. vulnificus
创伤弧菌(V. vulnificus)是一种重要的食源性病原体,能够导致坏死性伤口感染和原发性败血症(Baker-Austin C et al., 2017)。与副溶血弧菌相似,创伤弧菌相关感染有两个不同来源:食用受污染的海产品,特别是软体贝类,导致胃肠炎或原发性败血症;或伤口暴露于海水或海鲜产品,导致伤口感染和继发性败血症(Baker-Austin C et al., 2017)。创伤弧菌具有3 个生物型:生物Ⅰ型、生物Ⅱ型和生物Ⅲ型(Heng Sing-Peng et al., 2017)。创伤弧菌生物Ⅰ型是大多数伤口感染的主要原因;创伤弧菌生物Ⅱ型是养殖鳗鱼迅速致命败血症的病原体,在人类中很少发现(Fouz Belén et al., 2007);生物Ⅲ型被认为是生物Ⅰ型和Ⅱ型的杂交体(Heng Sing-Peng et al., 2017)。创伤弧菌的致病机制复杂,所致疾病为多种致病因子以及多个途径的共同作用(叶淑瑶等, 2018)。其毒力因子包括参与组织侵袭和鞭毛表达的tonB 系统、介导肠道定植的vvpE、由gbpA 编码的粘蛋白结合蛋白(Jang Kyung Ku et al., 2016)以及调节生物膜形成和毒力的双组分信号转导系统gacS-gacA 等(Gauthier Julie D et al., 2010)。
V. vulnificus is an important foodborne pathogen capable of causing necrotizing wound infections and primary sepsis (Baker-Austin C et al., 2017). Similar to V. parahemolyticus, V. vulnificus-associated infections have two distinct sources: consumption of contaminated seafood, particularly molluscan shellfish, which can lead to gastroenteritis or primary sepsis; or wound exposure to seawater or seafood products, which can lead to wound infection and secondary sepsis (Baker-Austin C et al., 2017). V. vulnificus has three biotypes: biotype I, biotype II, and biotype III (Heng Sing-Peng et al., 2017). The biotype I is the main cause of most wound infections; the biotype II is the pathogen of rapidly fatal sepsis in farmed eels and is rarely found in humans (Fouz Belén et al., 2007); and biotype III is considered to be a hybrid of biotypes I and II (Heng Sing-Peng et al., 2017). The pathogenic mechanism of V. vulnificus is complex and the related diseases are the results of various virulence factors through multiple pathways (Shuyao Ye et al., 2018). Its virulence factors include the tonB system, which is involved in tissue invasion and flagellar expression, vvpE, which mediates intestinal colonization, mucin-binding proteins encoded by gbpA (Jang Kyung Ku et al., 2016), and the two-component signaling system gacS-gacA, which regulates biofilm formation and virulence (Gauthier Julie D et al, 2010).
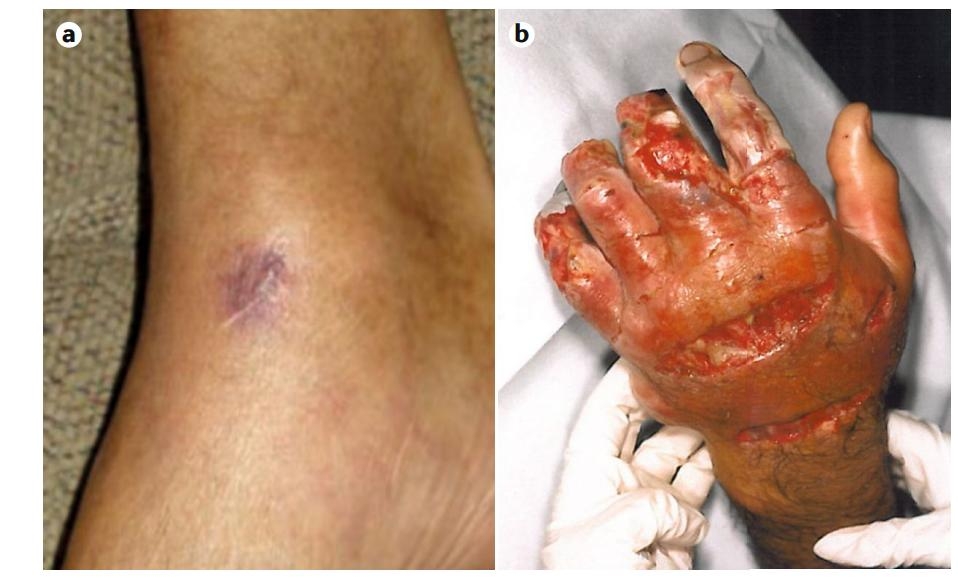
图3. 创伤弧菌引发的伤口感染
图片来源于美国北卡罗来纳大学J·D·奥利弗
Fig. 3. Wound infection caused by V. vulnificus
(J. D. Oliver, University of North Carolina, USA)
(5)霍乱弧菌
(5) V. cholerae
据统计,全世界每年约有300 万~500 万人感染霍乱弧菌(V. cholerae)(Jane N Zuckerman et al., 2007),因其感染导致的死亡人数高达10 万人每年。世界卫生组织数据显示,2022 年全球已有29 个国家和地区报告霍乱弧菌疫情。霍乱弧菌引发的感染在一些人口密度高、卫生条件差、缺乏饮用水的发展中国家非常常见。宿主摄入受污染的水或食物后,病原体沿小肠黏膜表面高密度增殖,但不会破坏上皮屏障的完整性或对上皮细胞造成实质性损害。但是霍乱弧菌会引起强烈的分泌反应,导致大量水样腹泻,如果不及时治疗,往往会在1-2 天内因脱水造成死亡(Baker-Austin C et al., 2017)。研究表明,霍乱弧菌的发病机制依赖于产毒菌株多种致病因子的协同作用。霍乱弧菌可以在小肠定植并产生肠毒素—霍乱毒素(Faruque et al., 1998);TCP(毒素调节菌毛)是霍乱弧菌在人类肠道定植所必需的;除此之外,LPS O-抗原、运动和代谢过程,都与霍乱弧菌在肠道的定植有关。
According to statistics, approximately 3-5 million people worldwide are infected with V. cholerae each year (Jane N Zuckerman et al., 2007), with up to 100,000 deaths per year. According to the World Health Organization, 29 countries and regions worldwide have reported V. cholerae outbreaks in 2022. Infections are common in developing countries with high population density, poor sanitation, and lack of access to drinking water. After ingestion of contaminated water or food by the host, the pathogen proliferates at high density along the mucosal surface of the small intestine without disrupting the integrity of the epithelial barrier or causing substantial damage to epithelial cells. However, V. cholerae causes a strong secretory response resulting in massive watery diarrhea and often death due to dehydration within 1-2 days if left untreated (Baker-Austin C et al., 2017). Studies have shown that the pathogenesis of V. cholerae relies on the synergistic effect of multiple pathogenic factors of strain. V. cholerae can colonize the small intestine and produce the enterotoxin which is the cholera toxin (Faruque et al., 1998). TCP (toxin coregulated pilus) is required for V. cholerae colonization in the human intestine. Besides, LPS O-antigen, motility and metabolic processes are all associated with V. cholerae colonization in the intestine.
主要参考文献
[1] Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J. Non-Cholera Vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25(1): 76-84.
[2] Baker-Austin C, Stockley L, Rangdale R, Martinez-Urtaza J. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: A European perspective. Environ Microbiol Rep 2: 7-18 [J]. PubMed, 2010.
[3] Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. [J]. Microbiology and molecular biology reviews: MMBR, 1998, 62(4).
[4] Fouz Belén, Roig Francisco J, Amaro Carmen. Phenotypic and genotypic characterization of a new fish-virulent Vibrio vulnificus serovar that lacks potential to infect humans. [J]. Microbiology (Reading, England), 2007, 153(Pt 6).
[5] Gauthier Julie D, Jones Melissa K, Thiaville Patrick, Joseph Jennifer L, Swain Rick A, Krediet Cory J, Gulig Paul A, Teplitski Max, Wright Anita C. Role of GacA in virulence of Vibrio vulnificus. [J]. Microbiology (Reading, England), 2010, 156(Pt 12).
[6] Grimes D Jay. The Vibrios: Scavengers, Symbionts, and Pathogens from the Sea. [J]. Microbial ecology, 2020, 80(3).
[7] Heng Sing-Peng, Letchumanan Vengadesh, Deng Chuan-Yan, Ab Mutalib Nurul-Syakima, Khan Tahir M, Chuah Lay-Hong, Chan Kok-Gan, Goh Bey-Hing, Pusparajah Priyia, Lee Learn-Han. Vibrio vulnificus: An Environmental and Clinical Burden. [J]. Frontiers in microbiology, 2017, 8.
[8] Jane N Zuckerman, Lars Rombo, Alain Fisch. The true burden and risk of cholera: implications for prevention and control [J]. The Lancet Infectious Diseases, 2007, 7(8).
[9] Jang Kyung Ku, Gil So Yeon, Lim Jong Gyu, Choi Sang Ho. Regulatory Characteristics of Vibrio vulnificus gbpA Gene Encoding a Mucin-binding Protein Essential for Pathogenesis. [J]. The Journal of biological chemistry, 2016, 291(11).
[10] Natividad-Bonifacio I, Fernández F J, Quiñones-Ramírez E I, Curiel-Quesada E, Vázquez-Salinas C. Presence of virulence markers in environmental Vibrio vulnificus strains. [J]. Journal of applied microbiology, 2013, 114(5).
[11] Prithvisagar Kattapuni Suresh, Krishna Kumar Ballamoole, Kodama Toshio, Rai Praveen, Iida Tetsuya, Karunasagar Iddya, Karunasagar Indrani. Whole genome analysis unveils genetic diversity and potential virulence determinants in Vibrio parahaemolyticus associated with disease outbreak among cultured Litopenaeus vannamei (Pacific white shrimp) in India. [J]. Virulence, 2021, 12(1).
[12] Sha Jian, Rosenzweig Jason A, Kozlova Elena V, Wang Shaofei, Erova Tatiana E, Kirtley Michelle L, Van Lier Christina J, Chopra Ashok K. Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis. [J]. Microbiology (Reading, England), 2013, 159(Pt 6).
[13] Zhang XH, Meaden PG, Austin B. Duplication of hemolysin genes in a virulent isolate of Vibrio harveyi. Appl Environ Microbiol, 2001, 67(7): 3161-3167.
[14] Zhang Xiao-Hua, He Xinxin, Austin Brian. Vibrio harveyi : a serious pathogen of fish and invertebrates in mariculture. [J]. Marine life science & technology, 2020, 2(3).
[15] 谢珍玉, 胡超群. 弧菌毒力基因水平转移与进化的研究进展 [J]. 热带海洋学报, 2005(03): 86-95.
[16] 梅冰, 周永灿, 徐先栋, 王世峰, 谢珍玉. 斜带石斑鱼烂尾病病原菌的分离与鉴定 [J]. 热带海洋学报, 2010, 29(06): 118-124.
[17] 夏凡, 杨丽君, 王静, 李兆杰, 刘玉敏. 病原性海洋弧菌致病机理及其快速检测方法研究进展 [J]. 食品工业科技, 2011, 32(01): 366-370+376. DOI: 10.13386/j.issn1002-0306. 2011. 01. 086.
[18] 俞盈, 张晏, 李俊, 杨虹, 宋厚辉, 方维焕. 副溶血弧菌 VPA1045 和VPA1049 调节 Hcp2 蛋白转位. 微生物学报, 2012, 52(8): 954–961.
[19] 叶淑瑶, 杨保伟, 李凤琴. 创伤弧菌致病性及致病机制研究进展 [J]. 中国食品卫生杂志, 2018, 30(02): 213-219.
References:
[1] Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J. Non-Cholera Vibrios: The microbial barometer of climate change. Trends Microbiol. 2017, 25(1): 76-84.
[2] Baker-Austin C, Stockley L, Rangdale R, Martinez-Urtaza J. Environmental occurrence and clinical impact of Vibrio vulnificus and Vibrio parahaemolyticus: A European perspective. Environ Microbiol Rep 2: 7-18 [J]. PubMed, 2010.
[3] Faruque S M, Albert M J, Mekalanos J J. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. [J]. Microbiology and molecular biology reviews: MMBR, 1998, 62(4).
[4] Fouz Belén, Roig Francisco J, Amaro Carmen. Phenotypic and genotypic characterization of a new fish-virulent Vibrio vulnificus serovar that lacks potential to infect humans. [J]. Microbiology (Reading, England), 2007, 153(Pt 6).
[5] Gauthier Julie D, Jones Melissa K, Thiaville Patrick, Joseph Jennifer L, Swain Rick A, Krediet Cory J, Gulig Paul A, Teplitski Max, Wright Anita C. Role of GacA in virulence of Vibrio vulnificus. [J]. Microbiology (Reading, England), 2010, 156(Pt 12).
[6] Grimes D Jay. The Vibrios: Scavengers, Symbionts, and Pathogens from the Sea. [J]. Microbial ecology, 2020, 80(3).
[7] Heng Sing-Peng, Letchumanan Vengadesh, Deng Chuan-Yan, Ab Mutalib Nurul-Syakima, Khan Tahir M, Chuah Lay-Hong, Chan Kok-Gan, Goh Bey-Hing, Pusparajah Priyia, Lee Learn-Han. Vibrio vulnificus: An Environmental and Clinical Burden. [J]. Frontiers in microbiology, 2017, 8.
[8] Jane N Zuckerman, Lars Rombo, Alain Fisch. The true burden and risk of cholera: implications for prevention and control [J]. The Lancet Infectious Diseases, 2007, 7(8).
[9] Jang Kyung Ku, Gil So Yeon, Lim Jong Gyu, Choi Sang Ho. Regulatory Characteristics of Vibrio vulnificus gbpA Gene Encoding a Mucin-binding Protein Essential for Pathogenesis. [J]. The Journal of biological chemistry, 2016, 291(11).
[10] Natividad-Bonifacio I, Fernández F J, Quiñones-Ramírez E I, Curiel-Quesada E, Vázquez-Salinas C. Presence of virulence markers in environmental Vibrio vulnificus strains. [J]. Journal of applied microbiology, 2013, 114(5).
[11] Prithvisagar Kattapuni Suresh, Krishna Kumar Ballamoole, Kodama Toshio, Rai Praveen, Iida Tetsuya, Karunasagar Iddya, Karunasagar Indrani. Whole genome analysis unveils genetic diversity and potential virulence determinants in Vibrio parahaemolyticus associated with disease outbreak among cultured Litopenaeus vannamei (Pacific white shrimp) in India. [J]. Virulence, 2021, 12(1).
[12] Sha Jian, Rosenzweig Jason A, Kozlova Elena V, Wang Shaofei, Erova Tatiana E, Kirtley Michelle L, Van Lier Christina J, Chopra Ashok K. Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis. [J]. Microbiology (Reading, England), 2013, 159(Pt 6).
[13] Zhang XH, Meaden PG, Austin B. Duplication of hemolysin genes in a virulent isolate of Vibrio harveyi. Appl Environ Microbiol, 2001, 67(7): 3161-3167.
[14] Zhang Xiao-Hua, He Xinxin, Austin Brian. Vibrio harveyi : a serious pathogen of fish and invertebrates in mariculture. [J]. Marine life science & technology, 2020, 2(3).
[15] Zhenyu Xie, Chaoqun Hu. Studying progress in horizontal transfer and evolution of Vibro virulence gene [J]. Journal of Tropical Oceanography, 2005(03): 86-95.
[16] Bing Mei, Yongcan Zhou, Xiandong Xu, Shifeng Wang, Zhenyu Xie. Isolation and identification of bacteria pathogens from Epinephelus coioides with tail-rotted disease [J]. Journal of Tropical Oceanography, 2010, 29(06): 118-124.
[17] Fan Xia, Lijun Yang, Jing Wang, Zhaojie Li, Yumin Liu. Research progress in pathogenetic mechanism and rapid detection of pathogenicm marine vibrio [J]. Science and Technology of Food Industry, 2011, 32(01): 366-370+376.
[18] Ying Yu, Yan Zhang, Jun Li, Hong Yang, Houhui Song, Weihuan Fang. VPA1045 and VPA1049 of Vibrio parahaemolyticus regulate translocation of Hcp2. Acta Microbiologica Sinica, 2012, 52(8): 954–961.
[19] Shuyao Ye, Baowei Yang, Fengqin Li. Research progress on pathogenicity and pathogenic mechanism of Vibrio vulnificus [J]. Chinese Journal of Food Hygiene, 2018, 30(02): 213-219.
 公众号
公众号