全球淡水资源极其有限,仅占总水量的3.5%左右,而海水资源丰富,如何利用丰富的海水实现能源循环利用变得尤其重要。海水淡化是解决饮用水资源短缺问题的一个重要途径,也是利用海水资源的一种可行方法,然而目前广泛使用的蒸馏法和膜处理法都存在成本过高的问题。另外,氢能作为新一代清洁能源,电解水制备氢是一种绿色且高效的方法,但是目前几乎所有的体系都使用淡水资源作为电解液,这无疑加剧了淡水资源短缺问题。如果直接电解海水产生氢气,其作为燃料又可产生高纯度淡水,可同时实现海水净化和产氢的双重目的。发展氢能经济是我国向可再生零碳能源结构转型,也是实现“碳达峰、碳中和”目标的必由之路。
Global freshwater resources are extremely limited, accounting for only about 3.5% of the total water volume, while seawater resources are abundant. Therefore, it has become particularly important to realize energy recycling using abundant seawater. Desalination is an important way to solve the shortage of drinking water resources and a feasible way to utilize seawater resources. However, the distillation method and membrane treatment method which are widely used at present have the problem of high cost. In addition, hydrogen energy is a new kind of clean energy, and the electrolysis of water to prepare hydrogen is a green and efficient method, but almost all current systems use freshwater resources as electrolyte, which undoubtedly aggravates the problem of freshwater resource shortage. If hydrogen used as fuel to produce high purity fresh water is produced directly by electrolysis of seawater, it can achieve the purposes of seawater purification and hydrogen production at the same time. The development of hydrogen economy is the way to transform China to a renewable zero-carbon energy nation and to achieve the goal of “carbon peak and carbon neutrality”.
2021年7月,大连理工大学精细化工国家重点实验室王治宇、邱介山教授与北京化工大学孙晓明教授合作,在《Nature Communications》发表题为“Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling hydrazine degradation”的研究论文,文章的中文译名是《无氯混合海水裂解耦合肼降解节能制氢》。该文基于对全电解水反应的解耦,提出了一种低能耗、无阳极氯腐蚀的混合海水电解制氢新技术。利用低氧化电位的肼氧化反应取代高能垒、反应动力学迟滞的阳极水氧化半反应,耦合工业含肼废水处理过程,实现了电解水制氢过程的高效节能降耗,并大幅降低制氢成本。
In July 2021, Prof. Wang Zhiyu and Qiu Jieshan from the State Key Laboratory of Fine Chemicals of Dalian University of Technology and Prof. Sun Xiaoming from Beijing University of Chemical Technology published a paper entitled “Energy-saving hydrogen production by chlorine-free hybrid seawater splitting coupling" in Nature Communications whose Chinese name is “无氯混合海水裂解耦合肼降解节能制氢”. Based on the decoupling of the all-electrolytic water reaction, the paper proposes a new technology of low-energy and anodic chlorine-free hybrid seawater electrolysis for hydrogen production. According to the paper, the energy-efficient and consumption-reducing electrolytic water hydrogen production process is realized and the cost of hydrogen production is significantly reduced by using the hydrazine oxidation reaction with low oxidation potential to replace the anodic water oxidation half-reaction with high energy barriers and hysteresis in reaction kinetics and by coupling the industrial hydrazine-containing wastewater treatment process.

阳极上的氯电氧化反应(ClOR)及其与析氧反应(OER)的竞争是一个突出的问题,该反应释放出有毒且具有腐蚀性的氯物种(如Cl2、ClO-),引发阳极溶解和环境危害,降低电解效率和损害可持续性。海水电解难以消除氯的交叉和腐蚀,且电池电压高(> 1.7-2.4 V),耗电量大(如图1)。迄今为止,发展无氯节能的海水电解技术仍然具有挑战性。
A notorious problem is the chlorine electro-oxidation reactions (ClOR) and their competition with oxygen evolution reaction (OER) on the anode. This reaction releases toxic and corrosive chlorine species (e.g., Cl2, ClO–), which induces anode dissolution and environmental hazards to reduce the electrolysis efficiency and sustainability. Nevertheless, it is still hard to eliminate the chlorine crossover and corrosion for long-term seawater electrolysis, and the process suffers high cell voltages (>1.7–2.4 V) and electricity consumption. So far, the development of chlorine-free yet energy-saving seawater electrolysis technology still remains challenging.

图1 混合海水裂解技术在节能和可持续制氢方面的示意图
在本文中,研究人员开发了一种耦合海水还原和热力学有利的肼氧化的有效策略。设计了一种NiCo/MXene基电极,该电极具有超疏水、亲水和肼友好的电催化界面(图2)。它可以全面提高界面电导率,稳定性,水/肼吸附能力和气体释放模式,以提高在大电流密度下的电解性能。混合海水电解槽可在0.7-1.0 V的超低电压下产生氢气,完全避免了在中性或碱性海水中氯对电池性能的危害。同时,在500 mA cm-2条件下稳定电解140 h,产氢速率为9.2 mol h-1gcat-1,法拉第效率高。相对于商用碱性水电解和最先进的海水电解器,在500 mA cm-2的高电流密度下,制氢基础能耗可降低30-52%。同时,肼以4.34±0.007 mol h-1gcat-1的快速降解速率快速降解至相当低的肼残留值~3 ppb。通过集成肼燃料电池或太阳能电池,实现了可持续性更好的自供电混合海水电解。
An efficient strategy coupling seawater reduction with thermodynamically favorable hydrazine oxidation is developed to address two extreme challenges of seawater electrolysis: the huge electricity consumption and notorious anode corrosion by chlorine chemistry. A NiCo/MXene-based electrode with superaerophobic-hydrophilic and hydrazine-friendly electrocatalytic interface is designed to fully exploit the potential of this chemistry. It allows for overall enhancement in interfacial conductivity, robustness, water/hydrazine adsorption capability and gas-releasing pattern for boosting electrolysis performance at large current densities. The hybrid seawater electrolyzer enables hydrogen production at ultralow cell voltages of 0.7–1.0 V, which fully avoids the chlorine hazards on cell performance in neutral or alkaline seawater. Meanwhile, the hydrogen can be produced at an intense rate of 9.2 mol h–1 gcat–1 by stable seawater electrolysis for 140 h at 500 mA cm–2 with high Faradaic efficiency. The electricity expense is largely reduced by 30–52% at a high current density of 500 mA cm–2 relative to commercial alkaline water electrolysis and the state-of-the-art seawater electrolyzers. Simultaneously, rapid hydrazine degradation to a rather low residual of ~3 ppb can be achieved at a fast rate of 4.34 ± 0.007 mol h–1 gcat–1. Self-powered hybrid seawater electrolysis is also realized by integrating hydrazine fuel cells or solar cells for hydrogen production with better sustainability.
 图2 NiCo@C/MXene/CF催化剂的制备示意图及其表征图
图2 NiCo@C/MXene/CF催化剂的制备示意图及其表征图
在高效制氢的同时,利用混合海水电解池阳极侧的肼氧化反应可快速降解水体中的剧毒肼污染物,避免了使用芬顿试剂等引入的二次污染。此类混合海水电解池可以方便与肼燃料电池或商业化太阳能电池连接,构建无需外部供电的自供能海水电解制氢装置,在AM 1.5G、100 mW cm-2太阳光辐照下,产氢速率可达6.0 mol h-1gcat-1。这一工作为发展低能耗、高经济和生态可持续性的低碳制氢技术方法提供了新的思路。使用肼污水作为阳极液和使用廉价的海水作为阴极液进料,可进一步降低氢成本。在此基础上,通过在沿海地区利用强烈的太阳照射和强风,将成本低廉的海水和工业肼污水注入可再生能源海水混合电解槽(HSE),可以扩大成本效益和可持续的制氢规模(图3)。
During the effective hydrogen production, the hydrazine oxidation reaction in the positive side of HSE can quickly dissolve the poisonous hydrazine pollutants in the waters, avoiding the second pollution of Fenton's reagents. Hydrogen production with better sustainability and cost-effectiveness can be realized by connecting the HSE into photovoltaic cells powered by easily harvestable and clean solar energy. Such a solar-driven hydrogen production system could be operated at a current density of ca. 310 mA cm–2 and an average photovoltage of ca. 0.876 V when powered by a single commercial solar cell (1.0 W). The hydrogen is yielded at a decent rate of 6.0 mol h–1 gcat–1 from seawater under AM 1.5 G simulated solar illumination with a power density of 100 mW cm–2. Using hydrazine sewage as the anolyte may in turn further reduce the hydrogen cost along with applying costless seawater as the catholyte feed. On this basis, cost-effective and sustainable hydrogen production might be scaled up by feeding costless seawater and industrial hydrazine sewage into renewables-powered HSE in the coastal region with intense solar irradiation and strong wind pattern.
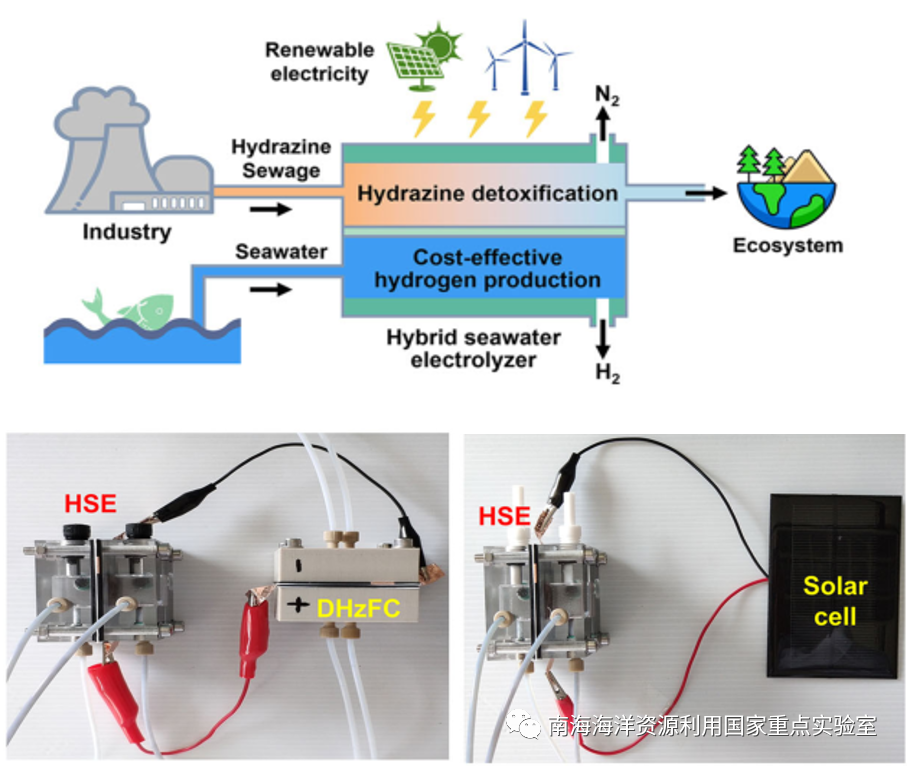
图3 可再生能源驱动的海水电解制氢-含肼工业废水处理联用工艺(上)
肼燃料电池或太阳能电池驱动的自供能海水电解制氢-肼降解双功能反应池(下)
研究人员认为可通过混合海水分解策略实现节能且无氯的海水电解,实现高效制氢。这种化学反应消耗阴极上的海水,通过析氢反应(HER)产生氢气;而释放的OH-到阳极侧的交叉将肼降解为无害的氮气和盐度降低的水。除了最先进的海水电解之外,它还可以在超低电池电压和大电流密度下生产氢气,而不会产生氯危害并限制产氢效率。
The researchers propose to realize energy-saving yet chlorine-free seawater electrolysis for efficient hydrogen production by a hybrid seawater splitting strategy. This chemistry consumes the seawater on the cathode to generate H2 by hydrogen evolution reaction (HER); while the crossover of released OH– to the anode side supply the hydrazine degradation to harmless N2 and water with reduced salinity. Beyond the state-of-the-art seawater electrolysis, it enables hydrogen production at ultralow cell voltages but large current densities without chlorine hazards and limiting hydrogen-yielding efficiency.
海水中存在的大量氯离子会造成阳极材料的严重腐蚀,进而导致电极损坏、电压过高。而如何延缓氯离子对阳极材料的腐蚀是海水电解制氢过程中需要解决的重点问题。
The large amount of chloride ions present in seawater can cause severe corrosion of anode materials, which in turn leads to electrode damage and high voltage. How to slow down the corrosion of anode materials by chloride ions is a key issue to be solved in the process of hydrogen production by seawater electrolysis.
总之,该工作对有效利用海洋资源以实现碳中和氢能经济的发展提供了非常有效的策略。
In conclusion, this work provides a fantastic strategy for the effective utilization of marine resources for the development of a carbon-neutral hydrogen energy economy.
参考文献:https://www.nature.com/articles/s41467-021-24529-3
简述翻译者:海南大学研究生王芳园
 公众号
公众号