LiNi0.6Co0.2Mn0.2O2(NCM622)具有充放电比容量高、来源丰富、环保无污染等优点而被认为是一种新一代高能量密度动力锂电池正极材料的理想之选。然而在高温下循环过程中所形成的晶间裂纹以及电极与电解液之间由于电解液分解所带来的副反应,会加速电极材料性能的衰减。为了解决这个问题,海南大学硅锆钛资源综合开发与利用重点实验室陈永教授课题组制备并研究了一系列TiO2改性LiNi0.6Co0.2Mn0.2O2(NCM622)正极材料。TiO2的加入减少了副反应的发生。此外,Ti4+由内到外的掺杂,强化了一次颗粒,减小了一次颗粒间的间隙,稳定了NCM622的充放电循环性能。掺入TiO2的NCM622表现出168.7 mAh g–1的高容量,200次循环后保持率为96%,比普通NCM622高出18%。结构和成分表征(即原位拉曼、EIS和深度XPS)进一步揭示了所设计的NCM622-TiO2正极材料的电化学和动力学机理。该文章发表在国际期刊Journal of power sources (一区,IF=7.467)。
LiNi0.6Co0.2Mn0.2O2(NCM622) is considered to be an ideal material for a new generation of high energy density lithium battery cathode with its advantages of enjoying high specific charge/discharge capacity, having abundant source, and protecting environment. However, the intergranular cracks formed during cycling at high temperatures and side reactions brought by the electrolyte decomposition between the electrode and electrolyte accelerate the degradation of electrode material performance. To resolve this issue, a series of TiO2-modified LiNi0.6Co0.2Mn0.2O2 (NCM622) cathode materials is constructed and studied. As expected, TiO2 could trap the residual lithium on the surface, minimizing the undesired side reaction. In addition, infiltrative layer with Ti gradient concentration from the outer to inner is also acquired, which strengthens the primary particles, reduces the gaps between randomly oriented grains and stabilizes the structure of NCM622 during charge discharge cycles. As a result, the NCM622 with TiO2 incorporation thus displays a high capacity of 168.7 mAh g−1, with 96% retention after 200 cycles, which is 18% higher than that of the bare NCM622. Structural and compositional characterization (i.e., in-situ Raman, EIS and depth XPS) further reveal the electrochemical mechanism and kinetics of the designed NCM622-TiO2 cathode. The paper was published in Journal of power sources (Ranking Q 1, IF=7.467).
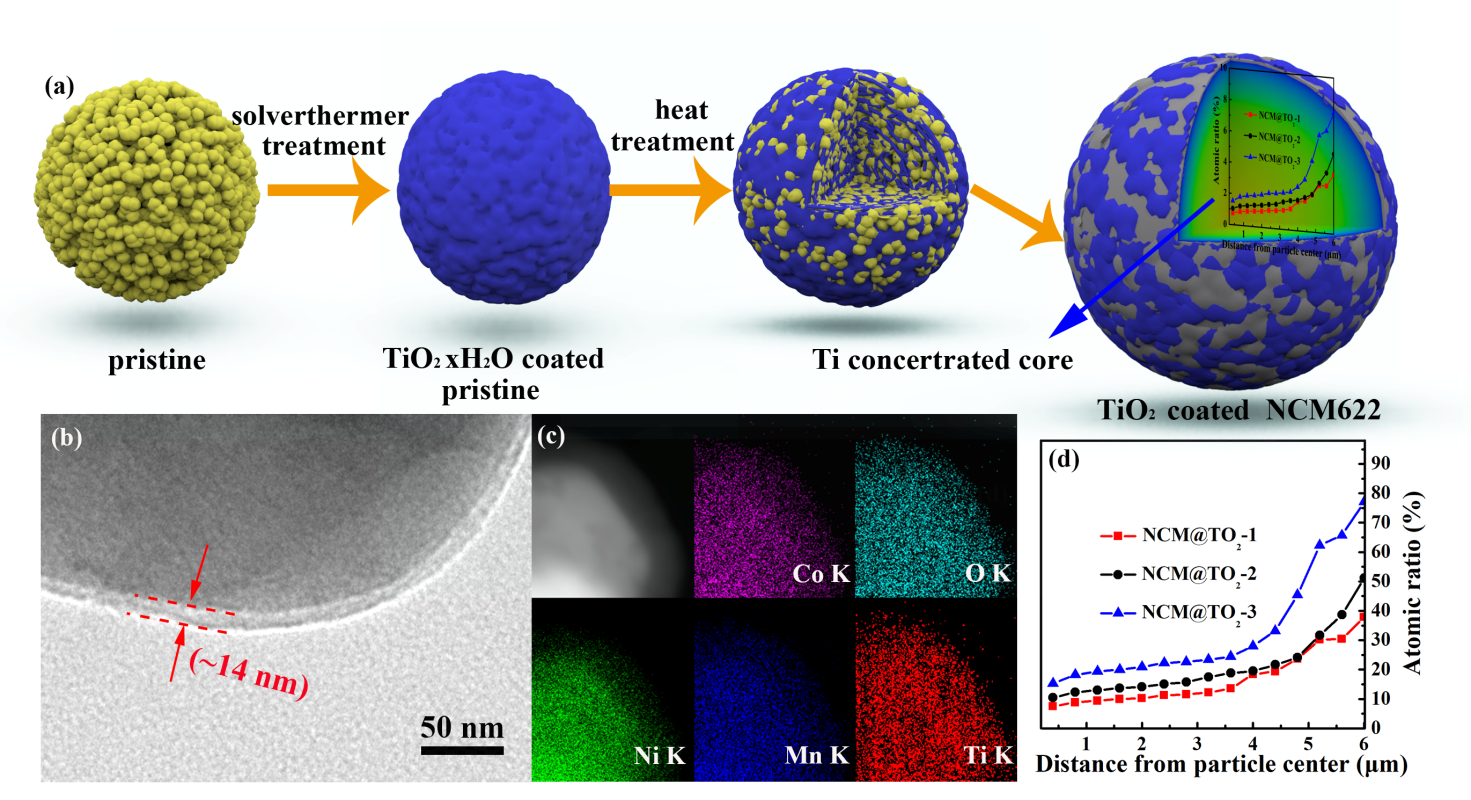
图1. NCM@TO2-x样品的合成路线(a);TEM图像(b)和相应的EDS结果(c);EPMA表征Ti含量的浓度梯度变化(d)。
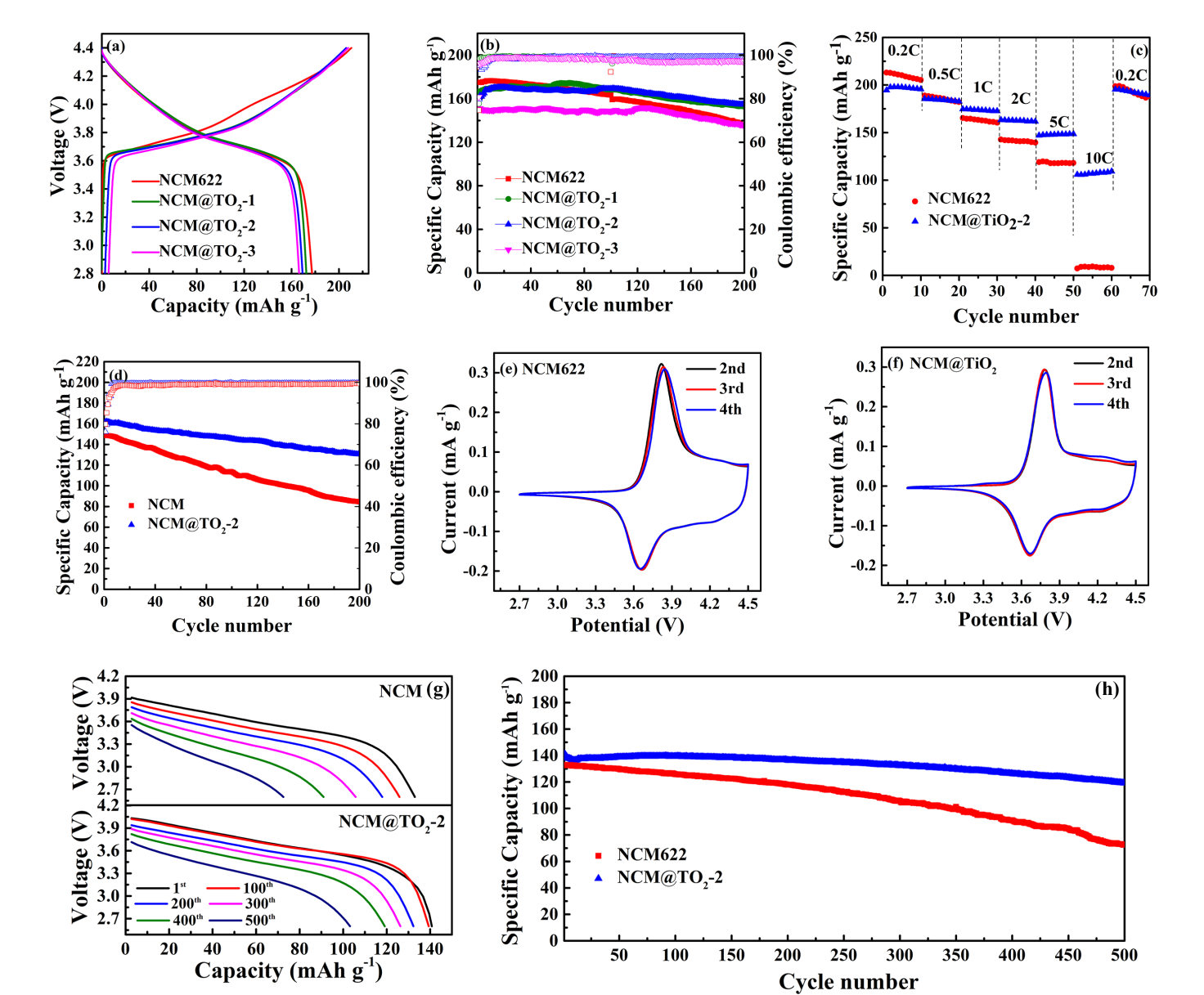
图2.在2.8-4.4 V(a-b)电压范围内1C时的初始充放电曲线和循环图;在不同充放电率(c)倍率性能;在2.8-4.6 V(d)电压范围内5C时的循环图;在0.1 mV s-1(e-f)下的CV;全电池在25℃下2.6-4.2 V放电曲线(a)和循环性能(b)的比较。
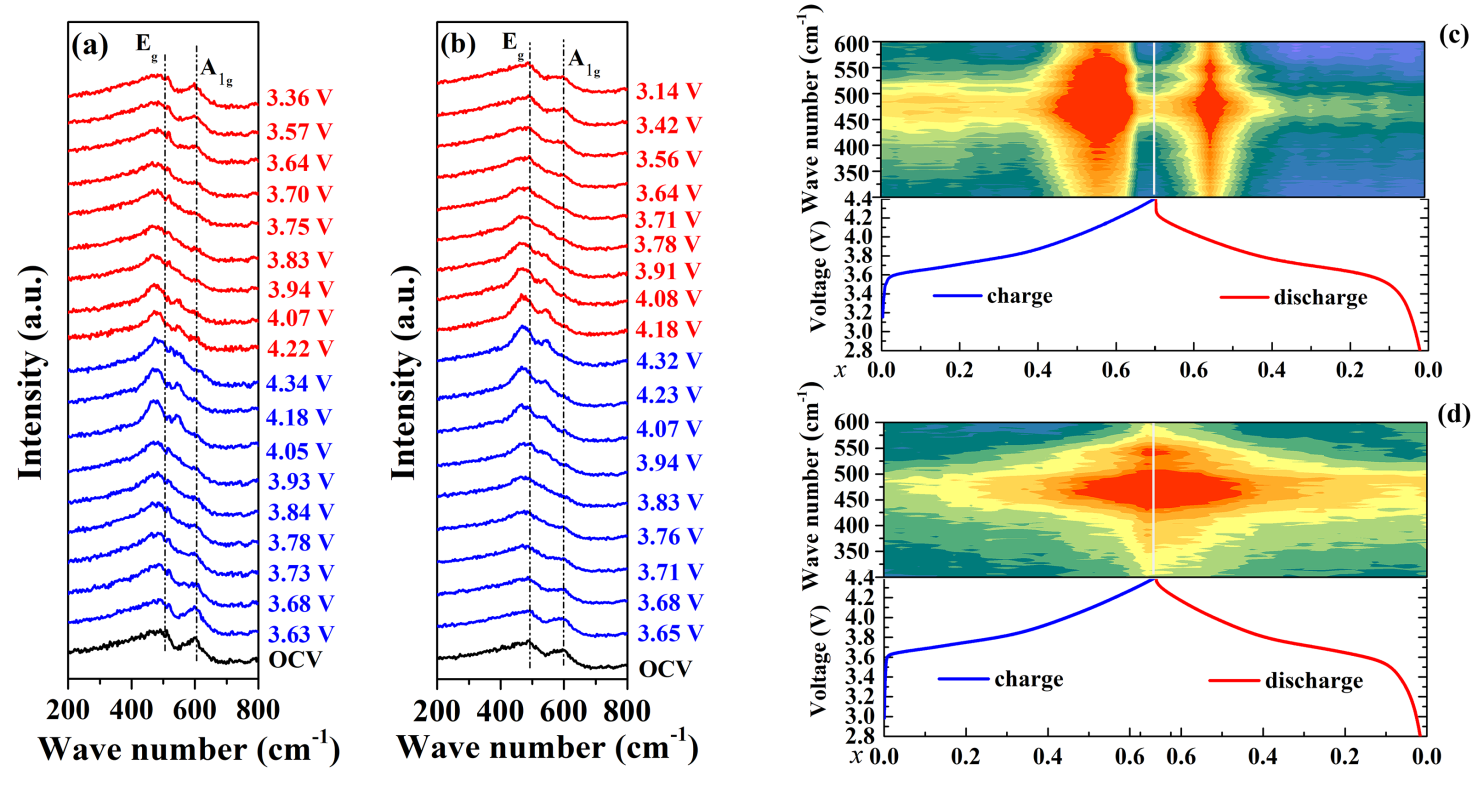
图3. NCM622 (a, c)和NCM@TO2-2 (b, d)的原位拉曼曲线。
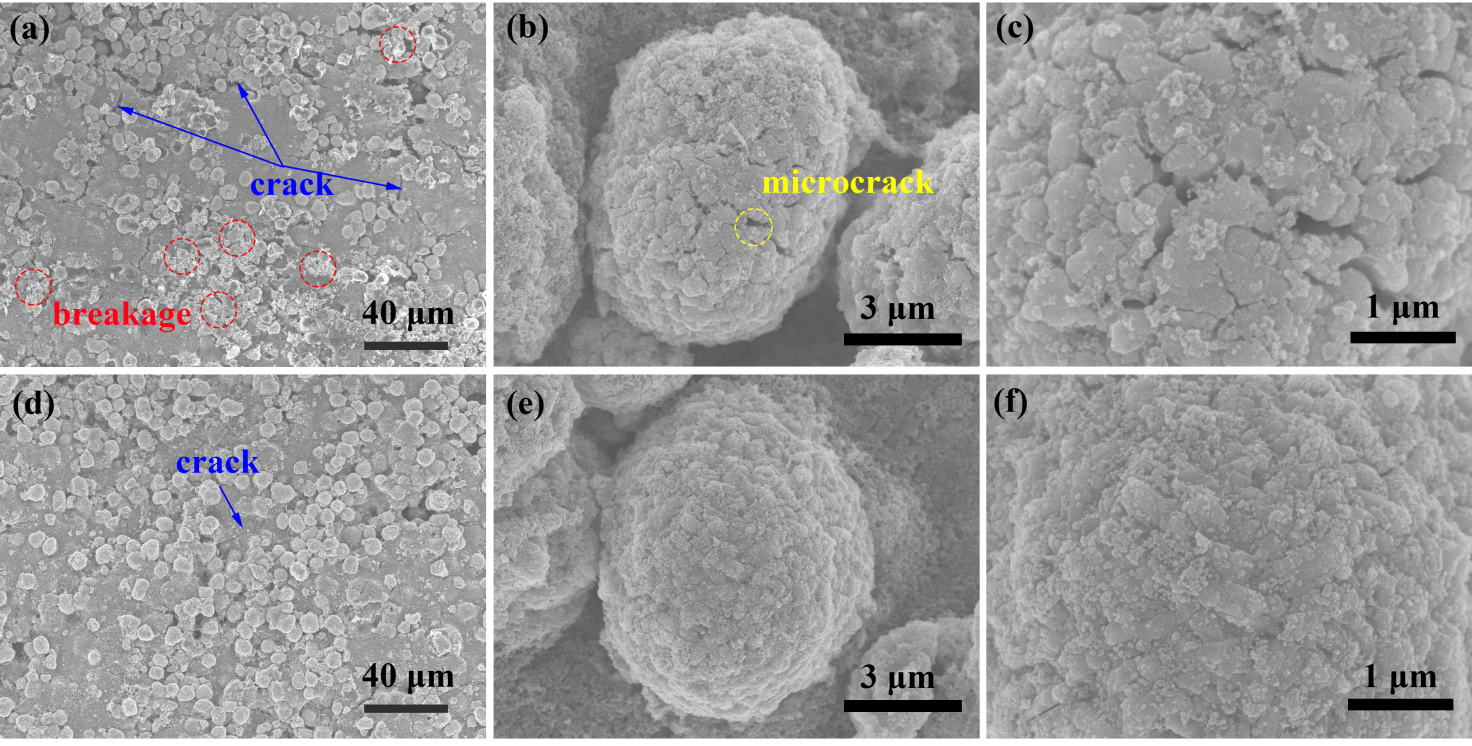
图4. NCM622 (a-c)和NCM@TO2-2(d-f)循环100圈后的极片SEM图。
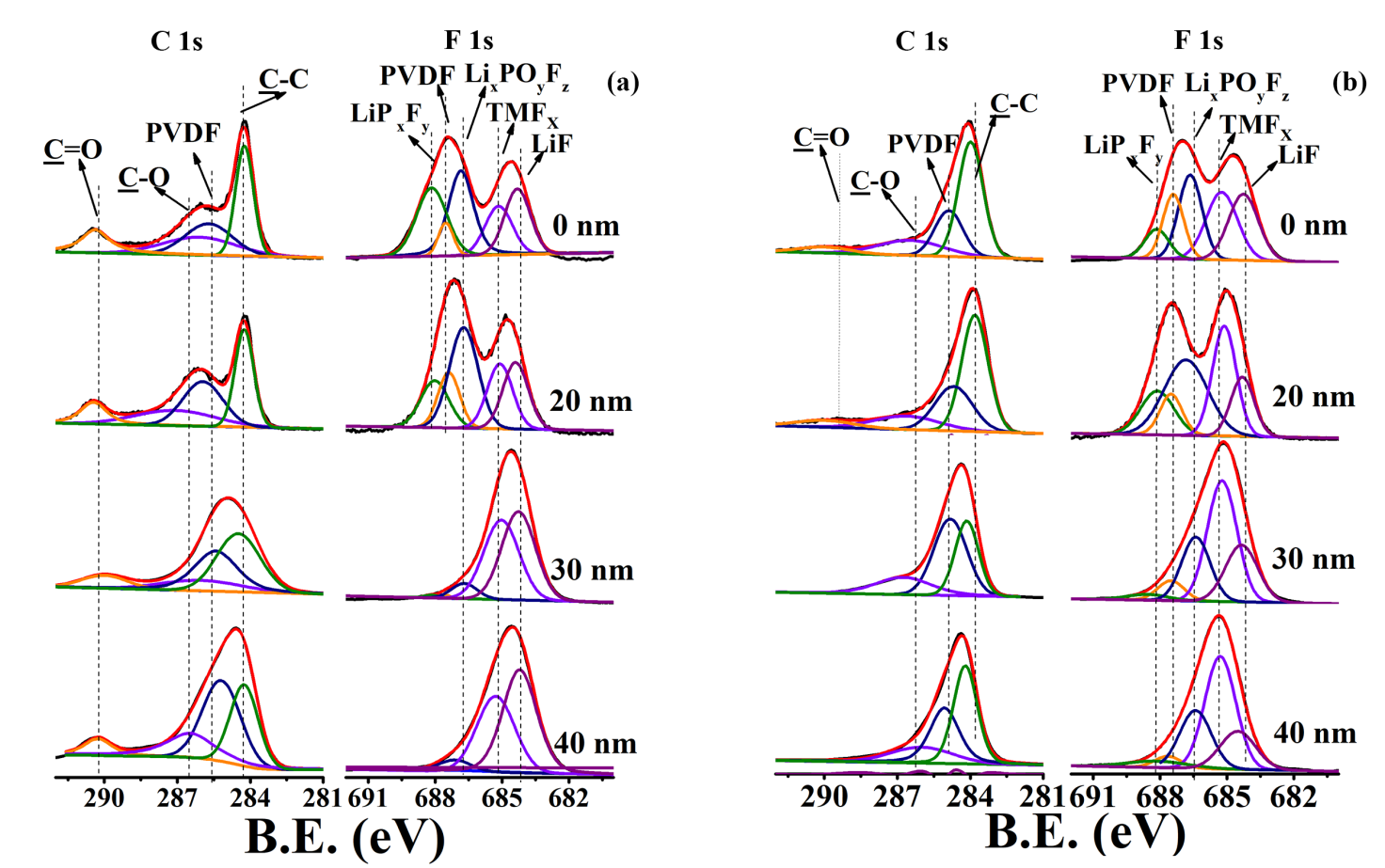
图5. NCM622 (a-c)和NCM@TO2-2 (d-f)循环100圈后的深度XPS
//www.hainanu.edu.cn
 公众号
公众号